CRO Service Area
Home > Our Markets > Pharmaceutical Contract R&D Service > CRO Service Area

Clinical research is an irreplaceable step in the pharmaceutical industry, and is also the stage with the most time and resources. It is the most important link in the whole pharmaceutical innovation system. Before drugs go on the market, in order to determine the safety and effectiveness of drugs, it is necessary to carry out drug research in human body; after drugs go on the market, it is also necessary to continue to carry out clinical research to increase indications or methods of administration and continuously observe the safety of drugs. Drug clinical trials are divided into Phase I clinical trial, Phase II clinical trial, Phase III clinical trial, Phase IV clinical trial and bioequivalence (BE) test. In 2016, the Opinions of the General Office of the State Council on Evaluation of Quality and Efficacy of Generic Drugs (GBF [2016] No. 8) was issued, which requires generic drugs to be approved for marketing before the implementation of new registration and classification of chemical drugs. A consistency evaluation on drugs must be carried out if they are not approved in accordance with the principle of consistent quality and efficacy with the original drugs. It is required that BE research is an indispensable link in the consistency evaluation of generic drugs (most oral preparations, some special injections and other dosage forms).
The clinical service team of the Life Sciences Division of Titanium Group is a clinical trial contract research organization (CRO) that provides high-quality and reliable services for drug clinical research. The company has an excellent clinical trial related technical team, including clinical operation, data management and statistical analysis, SMO and biological analysis teams, which can undertake clinical trial scheme design, clinical trial quality inspection, clinical data management, statistical analysis, SMO service, biological analysis, clinical trial audit and other services, aiming at providing "professional, standardized, high quality and reliable" integrated whole-process service in the quality and efficacy consistency evaluation of generic drugs for domestic pharmaceutical enterprises and clinical trial institutions, so as to making its own contribution to the development of China's pharmaceutical industry.
Medical writing
Clinical trial project management and quality supervision
Clinical data management
Statistical analysis
SMO service
Clinical trial audit
Bioanalysis
Project management
Meeting the requirements of all aspects of clinical research (from medical paper writing to data management), no more subcontracting and ensuring test quality in line with GCP requirements;
Transparent and reasonable price budgets, with clearly defined authority and accountability;
The research team has extensive experience, strategic planning and co-ordination capabilities;
A good industry reputation with customer satisfaction of 95% or above;
Quality management system
Normal SOP;
SOP for project management ;
SOP for project management ;
SOP for data analysis;
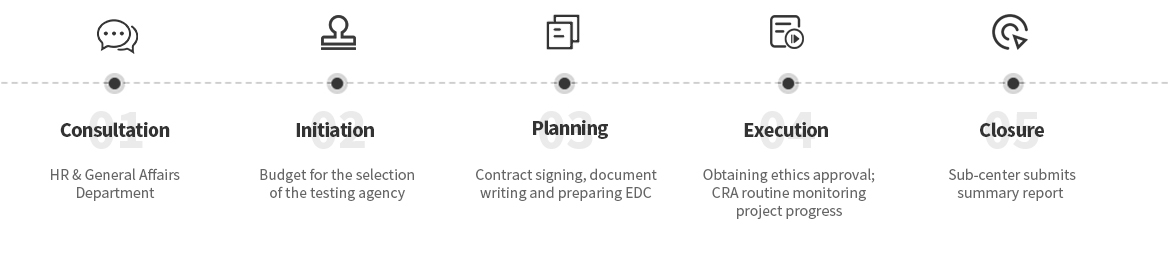
Consultation:HR & General Affairs Department
Initiation:Budget for the selection of the testing agency
Planning:Contract signing, document writing and preparing EDC
Execution:Obtaining ethics approval; CRA routine monitoring project progress
Closure:Sub-center submits summary report
Since its establishment, the clinical team has undertaken dozens of BE and pre BE projects, involving more than 20 product specifications, submitted more than 10 projects for registration, and passed seven projects with verification and exemption from verification; at the same time, it has undertaken SMO services for clinical trials of new drugs and devices; and has reserved dozens of drugs' schemes and methods.
Project situation
Pre-BE and BE turn-key project of a kind of statin dispersible tablets completed
Pre-BE and BE turn-key project of a kind of antibiotic capsule verified
BE turn-key project of a kind of antibiotic capsule completed
BE turn-key project of a kind of compound tablet completed
Pre-BE turn-key project of a kind of cardiovascular drugs completed
Formal BE turn-key project of a kind of gastrointestinal drugs to be verified/exempted from verification
Pre-BE turn-key project of a kind of cephalosporins completed
Formal BE turn-key project of a kind of anticoagulant tablet completed
Formal BE turn-key project of a kind of compound tablet verified
Formal BE turn-key project of a kind of Cephalosporin slow-release tablets to be verified/exempted from verification
Formal BE turn-key project of a kind of urinary drugs verified
Pre-BE turn-key project of a kind of medicine for hypertension completed
The company has long-term cooperation with a number of domestic pharmaceutical enterprises, with typical customers including Zhejiang Jinhua Conba Bio-pharm. Co., Ltd., Hangzhou Conba Bio-pharm. Co., Ltd., Zhejiang Anglikang Pharmaceutical Co., Ltd., Apeloa Pharmaceutical Co., Ltd., and Zhejiang Weicome Pharmaceutical Co., Ltd.; the cooperative medical institutions include Zhejiang Provincial People's Hospital and Sir Run Run Shaw Hospital affiliated to School of Medicine of Zhejiang University, The Affiliated Hospital of Hangzhou Normal University, The First Affiliated Hospital of Medical School of Zhejiang University, The Second Affiliated Hospital of Medical School of Zhejiang University, Hangzhou Combak Hospital, West China Second Hospital of Sichuan University, Jiangsu Province Hospital of Chinese Medicine, Nanjing First Hospital, etc., and successfully helped Wenzhou Hospital of Chinese Medicine to build Phase I clinical trial ward / bioequivalence research (BE) center.






