Bioanalytic testing services
Home > Our Services > Pharmaceutical Contract R&D Service > Bioanalytic testing services

Drug clinical trials are a complex process that selects the population to try to use the drugs for a certain period of time, and then through detection of the drug-like metabolic concentration in the body, the conclusion is drawn after statistics.
Medicine refers to chemical substances that may become drugs on the market, mostly mixtures. The National Drug Administration has strict requirements for the approval of such medicine as drugs. Whether the drug can be marketed, the laboratory for drug clinical trials is a key link .
Chemical and pharmaceutical bioanalysis
Evaluation of drug metabolism
Bioanalysis of pharmacodynamic biomarker
Detection of biomarkers in clinical diagnosis, precision medicine and functional medicine
The core management personnel have rich experience in GLP management and laboratory system management, and technical personnel are proficient in GLP requirements and receive annual GLP intensive training
The core instruments and equipment are calibrated, maintained and monitored according to GLP requirements, and corresponding identity data management files are established
The quality document SOP (Chinese and English) guides each step of the project developed from sample receiving to result reporting
The compliance of service items is supervised and guaranteed by the quality assurance department (QAU)
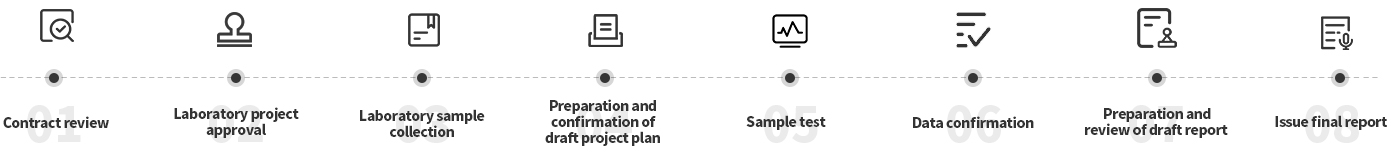
Contract review: Evaluate the resource allocation (personnel arrangement, material preparation) required to complete the task on time according to customer needs and actual conditions.
Laboratory project approval: The person in charge of the laboratory test unit shall assign the project leader according to the test arrangement
Laboratory sample collection: The person in charge of the test unit will allocate the samples to the project leader according to the test arrangement, and hand over other data and standard samples to the sample administrator for collection and storage;
Preparation and confirmation of the draft project plan: The project leader prepares the draft project plan according to the test application and submits it to the quality assurance department and the client for review and confirmation
Sample testing: Formal test is conducted after the project leader approves the project plan; the quality assurance department supervises the project process
Data confirmation, prepare draft report and review: The project leader reviews the test data, prepares the draft report, and submits it to the quality assurance department and the client for review
Issue the final report: The project leader shall sign and issue the final report. The original data and one final report shall be filed for 15 years, and other final reports shall be submitted to the client.




